- [131I]- Sodium Iodide solution
-
Product Code: IOM-1
- Description: Non-sterile 131I-Sodium Iodide solution containing sodium thiosulphate as oral solution
- Activity denominations:925 MBq-22.2 GBq
- Application: Treatment of thyroid related disorders
- Availability: Weekly production
- Expiry: 1 month from the calibration date
- Calibration: 3 to 5 days


- [131I]-Sodium Iodide capsules
-
Product Code: IOM-5
- Description: 131I-sodium Iodide loaded on gelatin capsules containing sodium sulphate dessicant
- Activity denominations: : 111, 185, 370, 925, 1850 and 3700 MBq capsules
- Application: Treatment of thyroid related disorders
- Availability: Weekly production
- Expiry: : 1 month from the calibration date
- Calibration: 3 to 5 days


- [131I]-m-Iodobenzylguanidine (mIBG) injection
-
Product Code: IOM-50T
- Description: Sterile 131I labelled meta-iodobenzylguanidine (131I-mIBG) in acetate buffer/normal saline containing 0.9% (v/v) benzyl alcohol as preservative
- Production method: Isotope exchange
- Activity denomination: 3.7 GBq
- Radiochemical purity : 95%
- Specific activity: 1.85-11.1 GBq/mg on calibration date
- Radioactive concentration: 185-555 MBq/ mL
- Application: Therapy of mIBG positive Pheochromocytoma, Paraganglioma, Neuroblastoma and Medullary carcinoma of thyroid
- Availability: Fortnightly production
- Expiry: Three days from the calibration date
- Calibration: 3 days
- Storage: Below -20⁰ C
- [177Lu]-Lutetium-177 Ethylene Diamine Tetramethylene Phosphonate (EDTMP) injection
-
Product Code: LUM-1
- Description: Sterile 177Lu labelled with EDTMP in carbonate buffer solution
- Activity denomination: 2.8 GBq
- Radioactive concentration: 370-740 MBq/mL on calibration date and time
- Radiochemical purity: >95%
- Application: Bone pain palliation due to metastases in advanced stages of breast/lung/prostate cancer
- Availability: Monthly production
- Expiry: 4 days from the day of production
- Calibration: 2 days
- Storage: Room temperature

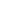
- [177 Lu]-Lutetium DOTA-TATE injection
-
Product Code : LUM-3
- Description: Sterile 177Lu labelled DOTA-TATE peptide in 0.2 M ammonium acetate buffer containing 0.1 M gentisic acid as radioprotectant
- Activity denomination: 3.7 and 7.4 GBq
- Radiochemical purity: >95%
- Specific activity: >26 GBq/mg on calibration date
- Radioactive concentration: 370-740 MBq/mL
- Application: Therapy of somatostatin receptor (sstr) positive neuroendocrine tumors
- Availability:Fortnightly production
- Expiry: 3 days from the calibration date
- Calibration: 2 days
- Storage: Below -20⁰C

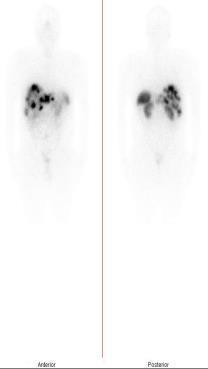
- [177Lu]-Lutetium PSMA-617 injection
-
Product Code: LUM-5
- Description: Sterile 177Lu labelled PSMA-617 peptide in 0.1 M sodium acetate buffer containing 2% ascobic acid as radioprotectant
- Activity denominations: 3.7 and 7.4 GBq
- Radiochemical purity: > 95%
- Specific activity: >26 GBq/mg on calibration date
- Radioactive concentration: 370-740 MBq/mL
- Application: Therapy of prostate cancer
- Availability: Monthly production
- Expiry: 3 days from the calibration date
- Calibration: 2 days
- Storage: Below -20⁰ C
- [153Sm]-Samarium Ethylene Diamine Tetramethylene Phosphonate (EDTMP) injection
-
Product Code: SAM-2
- Description: Sterile 153Sm labelled with EDTMP in saline solution
- Activity denomination: 2.8 GBq
- Radioactive concentration: 296-925 MBq/mL on calibration date and time
- Radiochemical purity: > 95%
- Application: Bone pain palliation due to metastases in advanced stages of breast/lung/prostate cancer
- Availability: Thrice in a month
- Expiry: 5 days from the production day
- Calibration: 2 days
- Storage: Room temperature
- Therapeutic Cold Kits
-
These kits are prepared under aseptic, sterile and pyrogen free conditions. When reconstituted with sterile, pyrogen-free [188Re]-Sodium perrhenate (for REK1) and 177LuCl3 (for LUK1) as per the prescribed procedure, the kits yield desirable yield of the therapeutic radiopharmaceutical
- Description: Sterile and pyrogen free glass vial containing inactive pharmaceutical ingredient in lyophilized powder form
- Availability: In stock
- Expiry: 1 Year
- Packages: 1 vial
- Storage: Under refrigerated conditions
Product Code
Product Code Kits Application LUK-1 177Lu-EDTMP Bone pain palliation