In brachytherapy, the radiation dose is applied to tumor by sealed sources. The sources are implanted or placed for a certain period of time to the tumor tissue itself or in its close vicinity.
BRIT has designed and developed Ir-192 based brachytherapy unit, Karknidon which is likely to be available in the market very soon. One such unit has been provided to Tata Memorial Centre for trial runs.
Ruthenium-106 Plaque Therapeutic
Ru-106 plaques brachytherapy cures eye cancers, salvage visions and saves life.
Plaque Brachytherapy in Eye Cancer Treatment Applications :
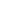
Plaque brachytherapy is a well proven modality for treatment of various eye cancers like uveal melanoma, retinoblastoma and choroidal melanomas.
Among the various radionuclides that can be used for plaque brachytherapy, Ru-106 plaques have gained wide popularity owing to a combination of unique radiochemical characteristics and excellent performance in terms of vision salvage. Further, the plaque needs no assembly and can be used as provided.
Ru-106 based brachytherapy delivers selective radiation dose emitted from its daughter Rh-106 (E = 3.54 MeV) as it decays to stable Pd-106, which minimizes damage to healthy tissues of the eye.
Importantly, the long half life of the parent radioisotope (Ru-106) makes the sources useful for treatment of multiple patients over a period of one year.
Offerings :
The Department of Atomic Energy (DAE) has dedicated two plaques for affordable eye cancer treatment.
| Product code | Descriptions | Diameter | Activity range |
|---|---|---|---|
| RUM1 | Ru-106 Round (A) Plaque | 15.8 mm | 15-37 MBq |
| RUM2 | Ru-106 Notched (N) Plaque | 21.0 mm | 20-50 MBq |
Plaques and accessories :
Each plaque comes with :
- One silver dummy plaque
- Certificate
- Dosimetry report
- Dose distribution profile

Ru-106 Round (A) Plaque :
Round plaque is extremely useful for the treatment of retinoblastoma, uveal and choroidal melonomas. The plaque is available with wide a range of activities which helps ophthalmologists select a suitable plaque depending upon the treatment required. Almost 40% of eye cancer patients can be treated using this plaque design.
Technical specification of the plaque is as follows :
- Material : Silver (99.9%)
- Outer diameter : 15.8 mm
- Active core diameter : 13.3 mm
- Spherical radius : 12 mm
- Total thickness : 1 mm
- Source strength : 15-37 MBq
- Useful life : 1 year
- Maximum uses : 50 times
- Source classification : : C 43312
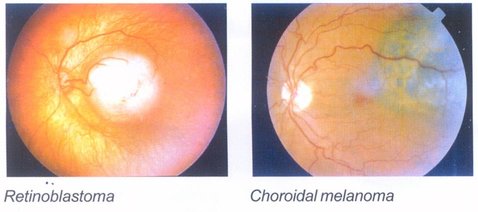

Dosimetry profile :
The dosimetry profile of each plaque is determined using a radiochromic film in a tissue equivalent phantom. The absorbed dose to water at different distances (in axial direction) from the plaque is measured. A typical dosimetry profile of a plaque is represented below for reference.
Additionally, dose distribution data both vertical and horizontal direction is generated by a computer simulation programme. The computed dose distribution not only assists doctors for planning the treatment, but also helps in estimating dose distribution to other parts of the eye.
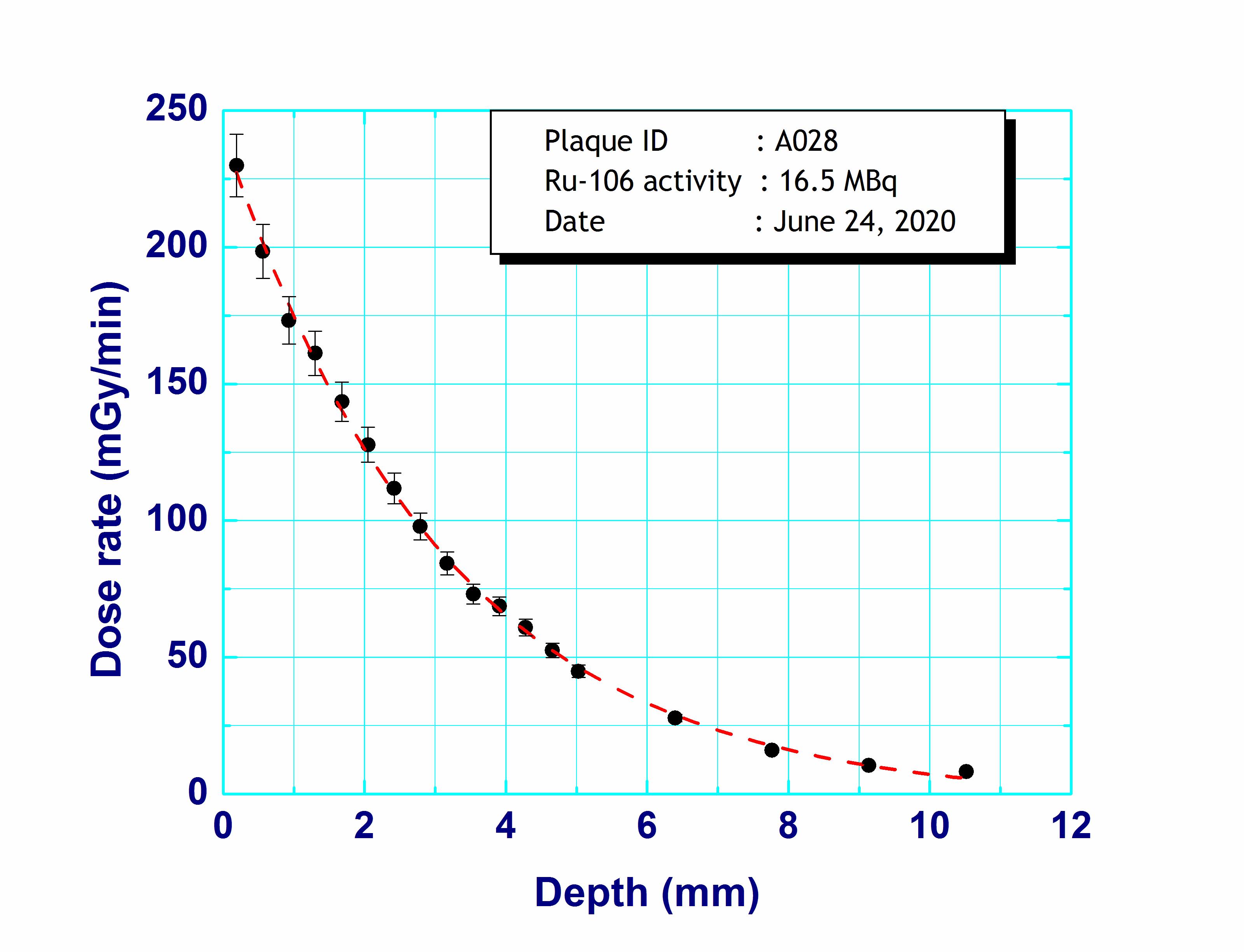
Ru-106 Notched (N) Plaque :
The notched plaque is developed for treatment of eye cancers located near the optical nerve. Unique design of the plaque facilitates selective dose delivery to the tumor while sparing the optical nerve, which is the most important part of the eye. The plaque is available with a wide range of activities, with low dose rates chosen smaller tumors, typically prevalent in children. The high active sources are useful for treatment of larger tumors upto 6 mm in height. High active plaques can be used for surface tumor treatment applications even beyond one year. Almost 50% of eye cancer patients can be treated with this plaque.
- Material : Silver (99.9%)
- Outer diameter : 21.0 mm
- Active core diameter : 18.5 mm
- Spherical radius : 12 mm
- Total thickness : 1 mm
- Source strength : 20-50 MBq
- Useful life : 1 year
- Maximum uses : 50 times
- Source classification : : C 43312

Highlights :
- Fission Ru-106 has been recovered in radiochemically pure form and used in the plaque preparation.
- Plaque manufacturing is done under stringent regulatory guidelines.
- Quality assurance tests for each plaque are done as per AERB SS3 guidelines endorsed by third party examination.
- The plaque can be sterilized by autoclave method after every use. Plaque integrity after 75 times of sterilization (autoclave conditions:121oC, 15 psi, 30 min) is ensured.
- Indigenous development of the plaque ensures affordable eye cancer treatment.
Ordering process :
- Apply for NOC (Product code: 21081 for Round (R) plaque and 21088 for Notched (N) plaque) from AERB through eLORA portal (https://www.aerb.gov.in)
- After obtaining NOC, order for product at www.britatom.gov.in
Customer review :
Click here for Customer Review